What Is the Product of the Hydrogenation of an Alkene
If both pi bonds are replaced with carbon-hydrogen bonds then an alkane forms. Alkenes are reduced to alkanes in the hydrogenation.

Alkenes Organic Chemistry Organic Chemistry Study Teaching Chemistry
Name one catalyst used in this reaction.
.jpg?revision=1&size=bestfit&width=409&height=109)
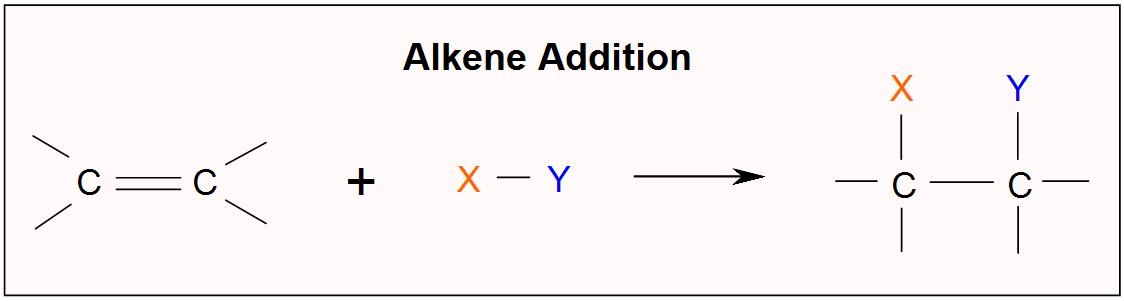
. An example of an alkene addition reaction is a process called hydrogenationIn a hydrogenation reaction two hydrogen. So the alkene is reduced by the addition of these two hydrogens. Alkane O dihaloalkane O ether O alcohol O haloalkane.
This is called hydrogenation. It includes the manufacture of. Why is 2 Bromopropane the major product.
What is the product formed. 84 What the product of the hydrogenation of an alkene. Examples of Catalytic Hydrogenation of Alkenes.
Ethane It forms ethane ie. Addition of X 2 to Alkenes. The first step in the addition of a hydrogen halide to an alkene is the dissociation of the hydrogen halide.
The only product involved in the hydrogenation of alkenes is the resulting alkane. See the answer See the answer done loading. And youll see other definitions for oxidation states.
One might assume that the reaction mechanism of the addition of. Halogenation and hydrogenation are examples of the addition of X 2-type molecules to alkenes. In this manner what is the product of the hydrogenation of an alkene.
If only one pi bond is replaced. The H ion is attracted to the πbond electrons of the. Who are the experts.
Ethylene ethene 2 H 2 CCH 2 is an alkene an unsaturated hydrocarbon. Youll see a gain in hydrogens is reduction. Hence we can say that ethane is the product of the hydrogenation of ethyne.
Alkene reacts with hydrogen gas in the presence of catalyst to give alkane as the product. Reactions of Alkenes and Alkynes. We call this reaction as hydrogenation of alkenes.
The product is an alkane. This page looks at the reaction of the carbon-carbon double bond in alkenes with hydrogen in the presence of a metal catalyst. This process yields semi-solid products like shortening and.
Hence according to Markovnikov Rule when hydrogen is added to the carbon with more hydrogen we will get the. In a hydrogenation reaction two hydrogen atoms are added across the double bond of an alkene. An example of an alkene addition reaction is a process called hydrogenation.
Hydrogenation is used in the food industry to convert liquid oils into saturated. An example of an alkene addition reaction is a process called hydrogenationIn a hydrogenation reaction two hydrogen atoms are added across the double bond of an alkene. Question What is the product of the hydrogenation of an alkene.
Chemistry questions and answers. An example of an alkene addition reaction is a process called hydrogenationIn a hydrogenation reaction two hydrogen atoms are added across the double bond of an alkene resulting in a. What is the product of the hydrogenation of ethyne.
What is the product from the reaction of an alkene with hydrogen. What is the product of the hydrogenation of an alkene. Hydrogenation is used in the food industry to convert liquid oils into saturated fats.
Alkenes and alkynes are generally more reactive than alkanes due to the electron density available in their pi bonds. Alkyne hydrogenation adds hydrogen atoms to the pi bond s. 84 What is the product of the hydrogenation of an alkene.
The catalyst is reformed so the reaction starts and ends with the same catalyst. The product is an alkane. It will undergo catalytic hydrogenation in the presence of a nickel.

Pin On Chemia Organiczna Schematy

Alkenes Organic Chemistry Study Organic Chemistry Chemistry Education
.jpg?revision=1&size=bestfit&width=409&height=109)
Catalytic Hydrogenation Of Alkenes Chemistry Libretexts

Hydrohalogenation Of Alkenes Chemistry Chemistry Class Organic Chemistry
No comments for "What Is the Product of the Hydrogenation of an Alkene"
Post a Comment